Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool
- Видео
- О видео
- Скачать
- Поделиться
Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool
785, 821 | 8 год. назад | 6, 332 - 0
Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool
In this video you are going to learn what the reaction rate is and some ways of measuring reaction rate.
Reaction rate is a measure of how quickly the reactants in a reaction change into the products of the reaction.
The rate of a chemical reaction can be measured in two ways:
1) The first way is to measure how quickly the reactants (the substances on the left of the arrow in the equation) decrease.
2) The second way is to measure how quickly the products (the substances on the right of the arrow in the equation) increase.
Example for 1: Measuring how quickly reactants decrease.
We will look at the reaction between marble chips (calcium carbonate) and hydrochloric acid.
As the reaction proceeds, the reactants lose mass because carbon dioxide gas is given off. We can measure the decrease in mass of reactants by using this type of apparatus with a conical flask stopwatch, cotton wool plug and balance.
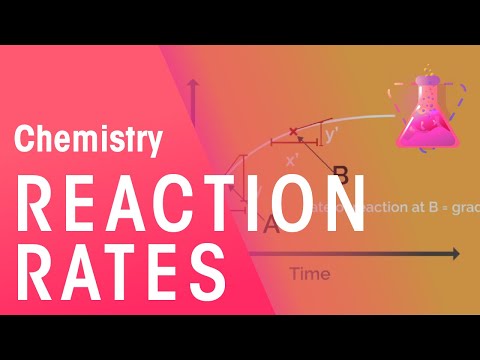
Based on our measurements, we plot a graph of ‘mass of reactants against time’.
The rate of reaction at point A is given by the gradient at A which is y/x and the rate of reaction at point B is given by the gradient at point B which is y’/x’ .
Example for 2:
Instead of measuring the loss of mass of the reactant we can also measure the gain in volume of the product carbon dioxide.
Watch part 2 to learn more:
SUPPORT US ON PATREON
SUBSCRIBE to the FuseSchool YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at www.fuseschool.org, where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here:
Find all of our Biology videos here:
Find all of our Physics videos here:
Find all of our Maths videos here:
Instagram:
Facebook:
Twitter:
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us:
Befriend us:
This is an Open Educational Resource. If you would like to use the video, please contact us: info@fuseschool.org
Чтобы скачать видео "Rates of Reactions - Part 1 | Reactions | Chemistry | FuseSchool" передвинте ползунок вправо
- Комментарии
Комментарии ФБ