Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
- Видео
- О видео
- Скачать
- Поделиться
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
781, 416 | 7 год. назад | 9, 143 - 0
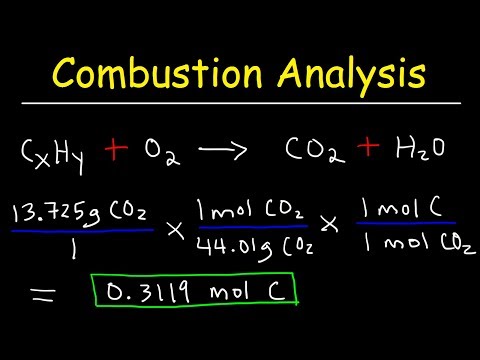
This chemistry video tutorial explains how to find the empirical formula and molecular formula using combustion analysis. It explains how to calculate the number of moles of each element given the mass in grams of CO2 and H2O. Examples include compounds containing Carbon, Hydrogen, and Oxygen. This video contains plenty of practice problems
Introduction to Moles:
How To Calculate The Molar Mass:
How To Convert Grams to Moles:
How To Convert Moles to Grams:
Moles to Atoms Conversion:
Grams to Molecules Conversion:
_________________________________
Grams to Atoms:
Moles, Atoms, & Grams Conversions:
How To Balance Chemical Equations:
Stoichiometry - Basic Introduction:
Avogadro's Number:
_________________________________
Limiting Reactant Problems:
Excess Reactant Problems:
Theoretical & Percent Yield:
Percent Yield - More Examples:
Percent Error:
_________________________________
Percent Composition By Mass:
Empirical Formula Problems:
Empirical Formula - Hydrated Compounds:
Combustion Analysis:
Stoichiometry Practice Test:
_______________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Stoichiometry Formula Sheet:
Чтобы скачать видео "Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems" передвинте ползунок вправо
- Комментарии
Комментарии ФБ